Fossil fuels have long been the precursor to plastic, but new research from the University of Nebraska–Lincoln and European collaborators could help send that era up in smoke — carbon dioxide, to be exact.
Produced almost entirely from burning fossil fuels, carbon dioxide concentrations in the atmosphere have risen from 280 parts per million in the pre-industrial era to about 410 PPM today. That trend, combined with the finite supply of fossil fuels, has pushed researchers to explore methods for producing plastic from CO2 rather than petroleum or natural gas — recycling CO2 just as plastic is now.
Nebraska’s Vitaly Alexandrov and colleagues have now detailed a catalyst-based technique that can double the amount of carbon dioxide converted to ethylene, an essential component of the world’s most common plastic, polyethylene.
“The conversion of CO2 is very important to help offset the emissions that lead to global warming and other detrimental processes in the environment,” said Alexandrov, assistant professor of chemical and biomolecular engineering.
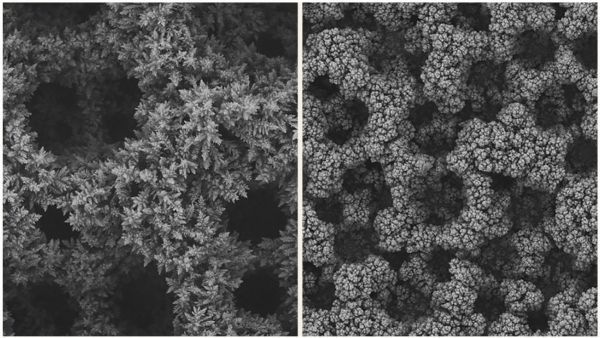
Copper has emerged as the prime candidate for catalyzing chemical reactions that convert carbon dioxide to plastic-forming polymer molecules, which it does when voltage is applied to it. But some copper-based setups have failed to convert more than about 15 percent of CO2 into ethylene, a yield too small to meet the needs of industry.
Read more at University of Nebraska–Lincoln
Image: Microscopic views of copper foam when untreated (left) vs. coated with a polymer called polyacrylamide, which new research has shown can double the conversion of CO2 to ethylene. CREDITS: American Chemical Society / ACS Catalysis