Led by the University of Manchester, an international team of scientists has developed a metal-organic framework material (MOF) that exhibits a selective, fully reversible and repeatable capability to remove nitrogen dioxide gas from the atmosphere in ambient conditions. This discovery, confirmed by researchers using neutron scattering at the Department of Energy’s Oak Ridge National Laboratory, could lead to air filtration technologies that cost-effectively capture and convert large quantities of targeted gases, including carbon dioxide and other greenhouse gases, to facilitate their long-term sequestration to help mitigate air pollution and global warming.
As reported in Nature Materials, the material denoted as MFM-300(Al) exhibited the first reversible, selective capture of nitrogen dioxide at ambient pressures and temperatures—at low concentrations—in the presence of moisture, sulfur dioxide and carbon dioxide. Despite the highly reactive nature of nitrogen dioxide, the MFM-300(Al) material proved extremely robust, demonstrating the capability to be fully regenerated, or degassed, multiple times without loss of crystallinity or porosity.
“This material is the first example of a metal-organic framework that exhibits a highly selective and fully reversible capability for repeated separation of nitrogen dioxide from the air, even in presence of water,” said Sihai Yang, one of the study’s lead authors and a lecturer in inorganic chemistry at Manchester’s School of Chemistry.
Professor Martin Schröder, another lead author from Manchester Chemistry, commented, “Other studies of different porous materials often found performance was degraded in subsequent cycles by the nitrogen dioxide, or that the regeneration process was too difficult and costly.”
Read more at DOE/Oak Ridge National Laboratory
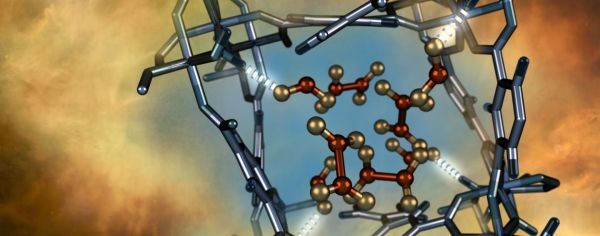
Image: Illustration of a nitrogen dioxide molecule (depicted in red and gold) confined within a nano-size pore of an MFM-300(Al) metal-organic framework material as characterized using neutron scattering at Oak Ridge National Laboratory. (Credit: ORNL/Jill Hemman)