For decades, scientists have searched for effective ways to remove excess carbon dioxide emissions from the air and recycle them into products such as renewable fuels. But the process of converting carbon dioxide into useful chemicals is tedious, expensive, and wasteful and thus not economically or environmentally viable.
Now a discovery by researchers at the U.S. Department of Energy’s Lawrence Berkeley National Laboratory (Berkeley Lab) and Joint Center for Artificial Photosynthesis (JCAP) shows that recycling carbon dioxide into valuable chemicals and fuels can be economical and efficient – all through a single copper catalyst.
The work appears in the Dec. 17 edition of the journal Nature Catalysis.
Going where the action is: product-specific active sites
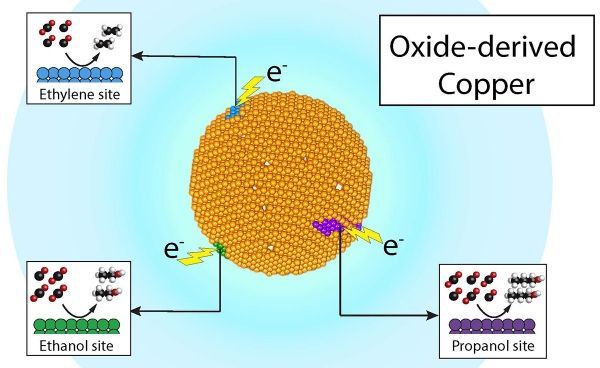
When you take a piece of copper metal, it may feel smooth to the touch, but at the microscopic level, the surface is actually bumpy – and these bumps are what scientists call “active sites,” said Joel Ager, a researcher at JCAP who led the study. Ager is a staff scientist in Berkeley Lab’s Materials Sciences Division and an adjunct professor in the Department of Materials Science and Engineering at UC Berkeley.
Read more at Berkeley Lab — Lawrence Berkeley National Laboratory
Image: Researchers at Berkeley Lab and the Joint Center for Artificial Photosynthesis have demonstrated that recycling carbon dioxide into valuable chemicals such as ethylene and propanol, and fuels such as ethanol, can be economical and efficient – all through product-specific “active sites” on a single copper catalyst. CREDIT: Joel Ager and Yanwei Lum/Berkeley Lab