Rice University scientists have given organic chemists a boost with their latest discovery of a one-step method to add nitrogen to compounds for drugs, pesticides, fertilizers and other products.
Rice synthetic organic chemist László Kürti said the method, reported in the Journal of the American Chemical Society, is a major step forward as it quickens and boosts the yield of valuable molecules known as alpha-aminoketones.
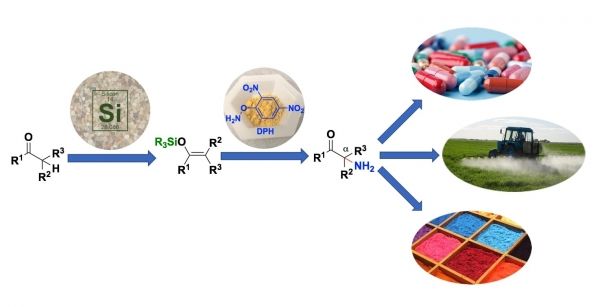
Ketones are carbon-based compounds found in nature and important feedstocks for the chemical industry. The primary amino group (NH2) is a functional group present in many important chemical products. It contains one nitrogen atom and two hydrogen atoms. When a ketone is functionalized with a primary amino group at the alpha carbon, it forms a compound called a primary alpha-aminoketone.
“It’s a good precursor, because there’s no extra functionalization, like an acyl group, on the NH2 and it can then be converted to whatever you want,” said Kürti, an associate professor of chemistry. “Previously, this was the issue: People would put nitrogen in there with extra functionality, but the further processing necessary to get to a free NH2 was complicated.”
Read more at Rice University
Illustration: Rice University chemists have discovered a one-step method to turn silicon-based silyl enol ether into nitrogen-bearing alpha-aminoketones, valuable building blocks in chemical design. CREDIT: Zhe Zhou