New data published this week in the Journal of Allergy and Clinical Immunology: In Practice suggests that oral immunotherapy is safe for preschool-aged children with peanut allergies.
The research, led by scientists and pediatric allergists at the University of British Columbia and BC Children’s Hospital, is the first to demonstrate the safety of peanut oral immunotherapy for a large group of preschool-aged children when offered as routine treatment in a hospital or clinic rather than within a clinical trial.
“Although there have been many clinical trials of peanut oral immunotherapy in older children, and one trial in preschoolers, there has been a lack of real-world data due to safety concerns of offering this treatment to preschoolers outside of a research setting,” said Lianne Soller, the study’s lead author and allergy research manager at BC Children’s Hospital. “But our findings confirm in a real-world setting that this treatment is not only safe but is well-tolerated in a large group of preschool-aged children.”
Oral immunotherapy (OIT) is a treatment protocol in which a patient consumes small amounts of an allergenic food, such as peanut, with the dose gradually increased to a determined maximum amount. The goal typically is to reach desensitization, where a patient can ingest more of the allergenic food without triggering a dangerous reaction—protecting them in the event of accidental exposure. Patients must continue to consume a determined amount of the allergen regularly as maintenance.
Read more at University of British Columbia
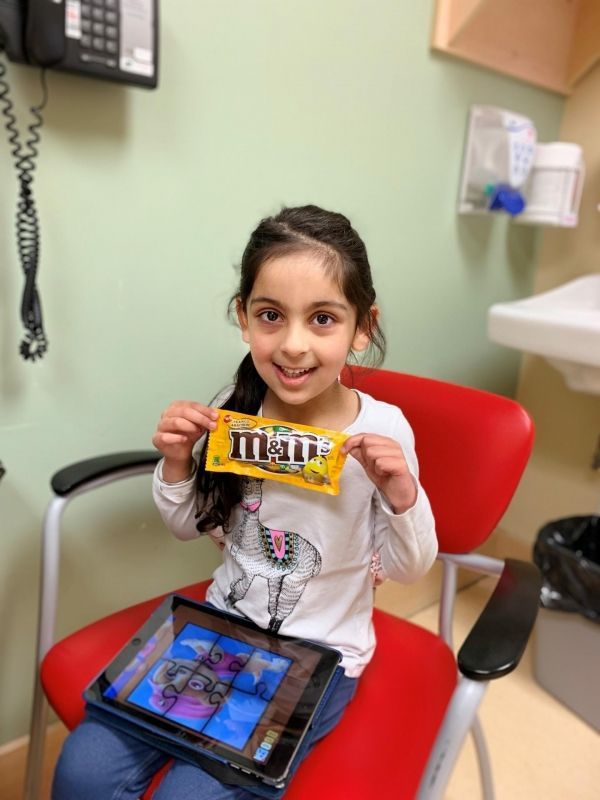
Image: Saiya Dhaliwal, 5, responded well to peanut OIT treatment. During an oral food challenge, she ate 10 peanut M&Ms without experiencing an allergic reaction. (Credit: Courtesy Ravinder Dhaliwal)