Researchers in Japan have found that the structure of Parkinson’s disease-associated protein aggregates can tell us, for the first time, about their movement through the brain. These new findings indicate that Parkinson’s disease is a kind of amyloidosis, which has implications for its diagnosis and treatment.
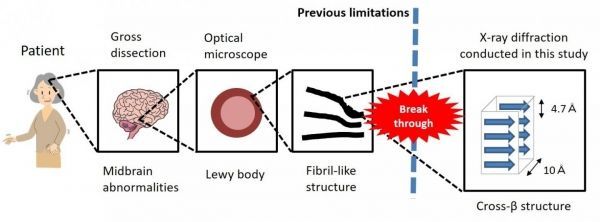
Lewy bodies, primarily composed of α-synuclein proteins (α-syn), are the neuropathological hallmark of Parkinson’s disease. However, we don’t yet fully understand how or why they appear in the brain. Using state-of-the-art imaging techniques, researchers at Osaka University have found that Lewy bodies in Parkinson’s disease brains contain α-syn protein aggregates (called amyloid fibrils) that can propagate through the brain. These findings, published this week in PNAS, support the new idea that Parkinson’s disease is a kind of amyloidosis, which is a group of rare diseases caused by abnormal protein accumulation.
“Our work follows on from in vitro findings that aggregates of α-synuclein that can propagate through the brain have a cross-β structure,” says lead author of the study Dr Hideki Mochizuki. “Our study is the first to find that aggregates in Parkinson’s disease brains also have this cross-β structure. This could mean that Parkinson’s disease is a kind of amyloidosis that features the accumulation of amyloid fibrils of α-synuclein.”
Read more at: Osaka University
Abnormal changes in the brains of patients with Parkinson's disease (Photo Credit: Osaka University)