One way to reduce the level of carbon dioxide in the atmosphere, which is now at its highest point in 800,000 years, would be to capture the potent greenhouse gas from the smokestacks of factories and power plants and use renewable energy to turn it into things we need, says Thomas Jaramillo.
As director of SUNCAT Center for Interface Science and Catalysis, a joint institute of Stanford University and the Department of Energy’s SLAC National Accelerator Laboratory, he’s in a position to help make that happen.
A major focus of SUNCAT research is finding ways to transform CO2 into chemicals, fuels, and other products, from methanol to plastics, detergents and synthetic natural gas. The production of these chemicals and materials from fossil fuel ingredients now accounts for 10% of global carbon emissions; the production of gasoline, diesel, and jet fuel accounts for much, much more.
“We have already emitted too much CO2, and we’re on track to continue emitting it for years, since 80% of the energy consumed worldwide today comes from fossil fuels,” says Stephanie Nitopi, whose SUNCAT research is the basis of her newly acquired Stanford PhD.
Read more at DOE/SLAC National Accelerator Laboratory
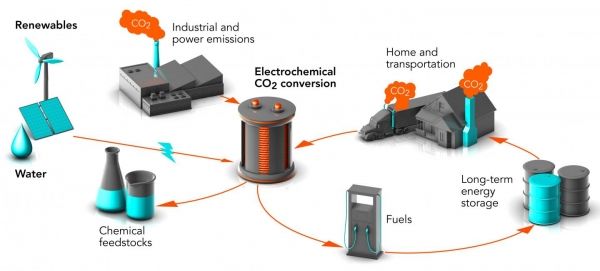
Image: Researchers at Stanford and SLAC are working on ways to convert waste carbon dioxide (CO2) into chemical feedstocks and fuels, turning a potent greenhouse gas into valuable products. The process is called electrochemical conversion. When powered by renewable energy sources, it could reduce levels of carbon dioxide in the air and store energy from these intermittent sources in a form that can be used any time. (Credit: Greg Stewart/SLAC National Accelerator Laboratory)