Ammonia, the primary ingredient in nitrogen-based fertilizers, has helped feed the world since World War I. But making ammonia at an industrial scale takes a lot of energy, and it accounts for more than one percent of the world’s total energy-related carbon emissions.
In nature, the enzyme nitrogenase produces ammonia in a much more environmentally benign way. Researchers are seeking to better understand how nitrogenase acts as a catalyst to break down nitrogen. What they learn could lead to new bio-inspired designs that improve the way fertilizers are made.
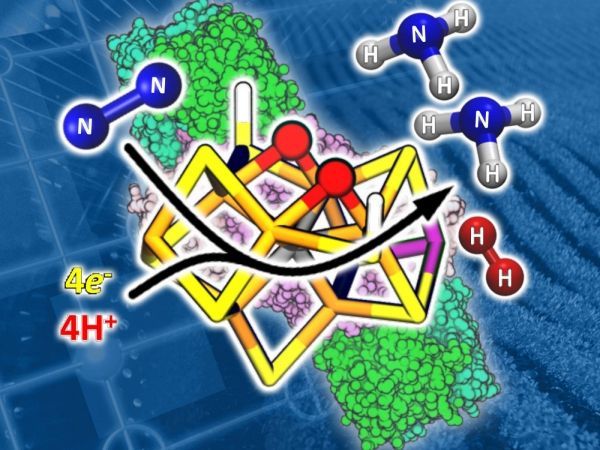
A recent discovery by a research team from PNNL and several universities crosses a huge hurdle toward that goal. They identified, for the first time, the elusive molecular structure inside nitrogenase that breaks down nitrogen to produce ammonia. This structure, referred to as the Janus intermediate, represents the turning point in the nitrogenase path toward ammonia.
Read more at Pacific Northwest National Laboratory
Image: Researchers from PNNL and several universities definitively identified the molecular structure of the Janus intermediate—a key supporting actor in the conversion of nitrogen into ammonia. The structure consists of two hydrides (red spheres) connecting two sets of iron ion pairs (orange rods). It binds a nitrogen molecule while eliminating hydrogen to form ammonia. CREDIT: Jeffrey London