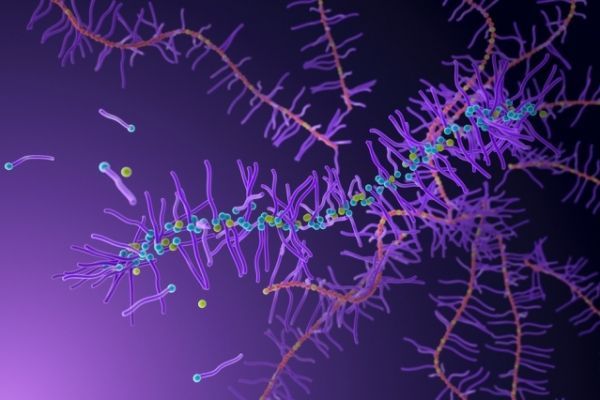
MIT chemists have devised a way to synthesize polymers that can break down more readily in the body and in the environment.
A chemical reaction called ring-opening metathesis polymerization, or ROMP, is handy for building novel polymers for various uses such as nanofabrication, high-performance resins, and delivering drugs or imaging agents. However, one downside to this synthesis method is that the resulting polymers do not naturally break down in natural environments, such as inside the body.
The MIT research team has come up with a way to make those polymers more degradable by adding a novel type of building block to the backbone of the polymer. This new building block, or monomer, forms chemical bonds that can be broken down by weak acids, bases, and ions such as fluoride.
“We believe that this is the first general way to produce ROMP polymers with facile degradability under biologically relevant conditions,” says Jeremiah Johnson, an associate professor of chemistry at MIT and the senior author of the study. “The nice part is that it works using the standard ROMP workflow; you just need to sprinkle in the new monomer, making it very convenient.”
Read more at Massachusetts Institute of Technology
Image: A new type of polymer designed by MIT chemists incorporates a special monomer (yellow) that helps the polymers to break down more easily under certain conditions. CREDIT: Demin Liu