A research group led by Associate Professor Takashi Tachikawa of Kobe University’s Molecular Photoscience Research Center has succeeded in developing photocatalysts that can convert an efficient level of hydrogen from water using solar light. It is hoped that methods like this one, which uses titanium-modified hematite mesocrystal-based photoanodes, could form the foundation for a commercial solar water splitting system. This would allow the clean fuel hydrogen to be produced more cheaply and easily than before, making it a viable source of renewable energy.
This was a joint research project with Nagoya University’s Institute of Materials and Systems for Sustainability (Professor Shunsuke Muto) and the Japan Synchrotron Radiation Research Institute (JASRI) (Chief Researchers Koji Ohara and Kunihisa Sugimoto).
The results of this study were first published in the online journal Nature Communications on October 23 2019.
Read more at Kobe University
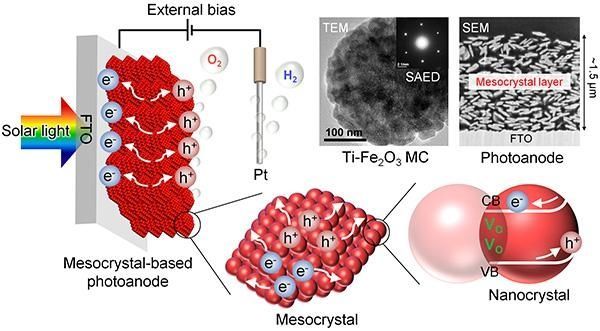
Image: Figure 3: The PEC water splitting method and the nanoparticle structure of the mesocrystal-based photoanodes The above diagram shows oxygen vacancies (Vo), and the movement of electrons (e-) and holes (h+). Using TEM (Transmission Electron Microscopy), it is possible to see the arrangement of nanoparticles inside the mesocrystals. SAED (Selected Area Electron Diffraction) was also carried out in order to examine the structure of the mesocrystals in more detail- indicating that the nanocrystals inside are highly ordered and aligned. The SEM image of the mesocrystal layer shows the disc-shaped mesocrystals and the network of pores and particles that aid light absorption and charge mobility, respectively. (Credit: Kobe University)