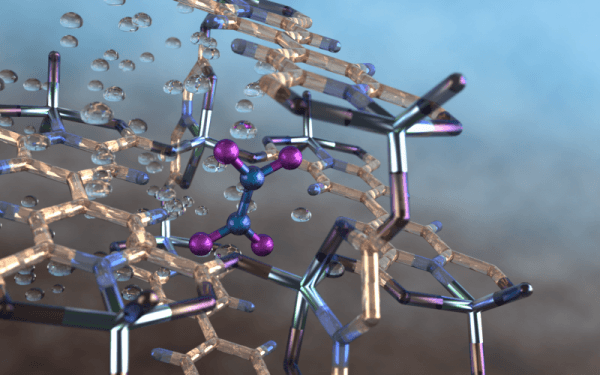
An international team of scientists, led by the University of Manchester, has developed a metal-organic framework, or MOF, material that provides a selective, fully reversible and repeatable capability to capture a toxic air pollutant, nitrogen dioxide, produced by combusting diesel and other fossil fuels.
The material then requires only water and air to convert the captured gas into nitric acid for industrial use. The mechanism for the record-breaking gas uptake by the MOF, characterized by researchers using neutron scattering at the Department of Energy’s Oak Ridge National Laboratory, could lead to air pollution control and remediation technologies that cost-effectively remove the pollutant from the air and convert itinto nitric acid for use in producing fertilizer, rocket propellant, nylon and other products.
As reported in Nature Chemistry, the material, denoted as MFM-520, can capture atmospheric nitrogen dioxide at ambient pressures and temperatures—even at low concentrations and during flow—in the presence of moisture, sulfur dioxide and carbon dioxide. Despite the highly reactive nature of the pollutant, MFM-520 proved capable of being fully regenerated multiple times by degassing or by treatment with water from the air—a process that also converts the nitrogen dioxide into nitric acid.
Read more at Oak Ridge National Laboratory
Image: Illustration of a nitrogen dioxide molecule (depicted in blue and purple) captured in a nano-size pore of an MFM-520 metal-organic framework material as observed using neutron vibrational spectroscopy at Oak Ridge National Laboratory. Image credit: ORNL/Jill Hemman