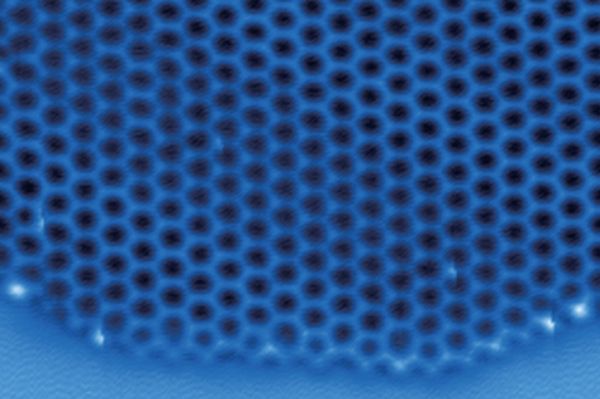
On frigid days, water vapor in the air can transform directly into solid ice, depositing a thin layer on surfaces such as a windowpane or car windshield. Though commonplace, this process is one that has kept physicists and chemists busy figuring out the details for decades.
In a new Nature paper, an international team of scientists describe the first-ever visualization of the atomic structure of two-dimensional ice as it formed. Insights from the findings, which were driven by computer simulations that inspired experimental work, may one day inform the design of materials that make ice removal a simpler and less costly process.
“One of the things that I find very exciting is that this challenges the traditional view of how ice grows,” says Joseph S. Francisco, an atmospheric chemist at the University of Pennsylvania and an author on the paper.
“Knowing the structure is very important,” adds coauthor Chongqin Zhu, a postdoctoral fellow in Francisco’s group who led much of the computational work for the study. “Low-dimensional water is ubiquitous in nature and plays a critical role in an incredibly broad spectrum of sciences, including materials science, chemistry, biology, and atmospheric science.
Read more at University of Pennsylvania
Image: An international team of scientists, including atmospheric chemists from Penn, describe the first-ever visualization of the atomic structure of two-dimensional ice as it formed. (Image: Courtesy of Joseph Francisco)