Evaporation ponds, which are commonly used in many industries to manage wastewater, can span acres, occupying a large footprint and often posing risks to birds and other wildlife. Yet they're an economical way to deal with contaminated water because they take advantage of natural evaporation under sunlight to reduce large volumes of dirty water to much smaller volumes of solid waste.
Now researchers at the Department of Energy's Lawrence Berkeley National Laboratory (Berkeley Lab) have demonstrated a way to double the rate of evaporation by using solar energy and taking advantage of water's inherent properties. The study, led by Berkeley Lab scientists Akanksha Menon and Ravi Prasher, is reported today in the journal Nature Sustainability.
Evaporation ponds are used at power plants, desalination plants, in the oil and gas industry, and also for lithium extraction, in which lithium-rich brine is pumped into vast, man-made salt ponds. They're common in China, Australia, Europe, the Middle East, and parts of the United States where the climate is suitable (arid or semi-arid with a lot of sunshine), and these ponds can be the size of hundreds of football fields, with many of them sitting side by side.
"This is a big societal problem we're trying to solve. To either dispose of the wastewater or to extract a valuable salt like lithium, you would like to increase the evaporation rate dramatically and in a scalable manner," said Prasher, an expert in thermal energy who also serves as Berkeley Lab's associate director for the Energy Technologies Area (https://eta.lbl.gov/). "If we could do so, that could reduce their environmental impact by reducing the amount of land required."
Read more at DOE/Lawrence Berkeley National Laboratory
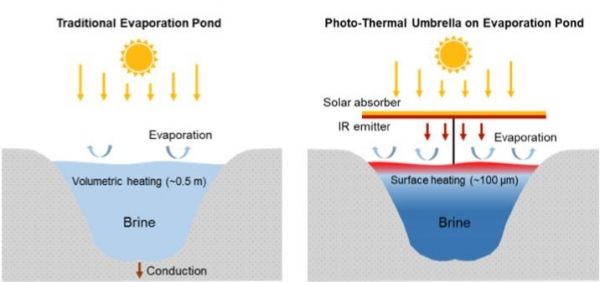
Image: In a conventional evaporation pond (left), incoming sunlight is absorbed, causing a bulk water temperature increase that leads to evaporation. With Berkeley Lab's proposed solar umbrella, incoming sunlight is converted into mid-infrared radiation, where water is strongly absorbing, thereby increasing surface temperature and evaporation rate while the bulk remains at a lower temperature. (Credit: Berkeley Lab)