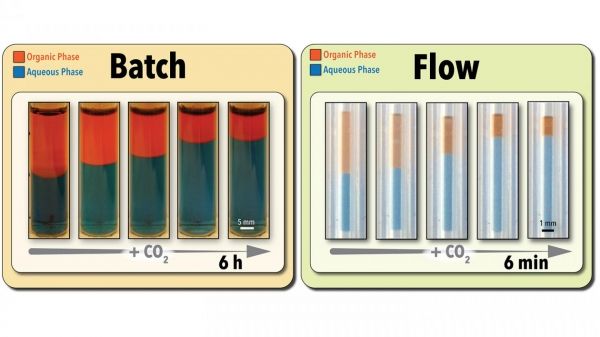
Researchers at North Carolina State University have demonstrated a new, green technology for both accelerated screening and retrieving “switchable” solvents used in green chemistry applications. The new approach makes the screening process hundreds of times faster and drastically accelerates the rate at which solvents can be retrieved from solution.
“We have effectively created a platform that makes green chemistry greener,” says Milad Abolhasani, an assistant professor of chemical and biomolecular engineering at NC State and corresponding author of a paper on the work. “This work expedites industry’s ability to identify the best switchable solvent for a specific chemical process and then gives industry advanced tools to retrieve that solvent far more quickly than is possible using previous approaches.”
At issue are switchable solvents, which change their physicochemical properties when exposed to carbon dioxide (CO2). This study focused on solvents that become hydrophilic in the presence of CO2 and water, but are hydrophobic when CO2 is removed. This makes them attractive for use in chemical and pharmaceutical industry processes, because the solvent can be easily removed from the product by adding CO2 and water. The solvent can then be reclaimed from the water by removing the CO2.
“However, from an industrial point of view, there are significant challenges,” Abolhasani says. “Specifically, the process for screening candidates to identify the most efficient switchable solvent for a particular application can be extremely time- and labor-intensive. And once you have the right switchable solvent candidate, removing it on a large scale can also take a long time.”
Read more at North Carolina State University
Image: Researchers at North Carolina State University have demonstrated a new, green technology for both accelerated screening and retrieving "switchable" solvents used in green chemistry applications. The new approach makes the screening process much faster than conventional batch testing techniques (as shown above) and drastically accelerates the rate at which solvents can be retrieved from solution. (Credit: Milad Abolhasani, NC State University)