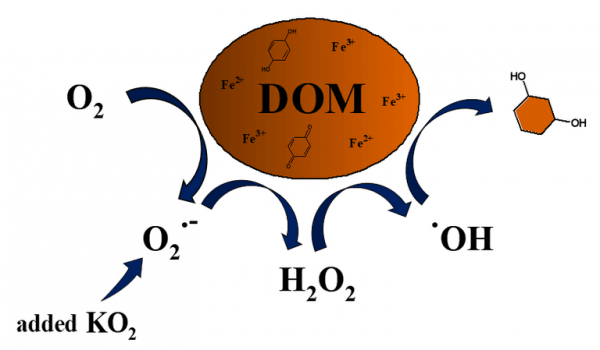
According to a study published in Water Research in April 2020, superoxide produces hydroxyl radicals in lake water. Hydroxyl radicals break down poorly biodegradable organic matter such as humic substances and anthropogenic pollutants.
In the aquatic environment, microbes, light and reduced compounds produce superoxide. Superoxide is a reactive oxygen species but relatively unreactive against organic compounds in water despite the prefix 'super' in its name.
Superoxide however can initiate a pathway of redox reactions. It can reduce ferric iron to ferrous one or be reduced itself to hydrogen peroxide. The Fenton reaction between ferrous iron and hydrogen peroxide produces hydroxyl radicals, very effective oxidants of organic matter. According the above mentioned reaction pathway, the production of one hydroxyl radical requires three superoxide ions.
Read more at Jyväskylä University
Image: Superoxide can drive autocatalytic production of hydroxyl radicals in the presence of complexes of natural dissolved organic matter and iron. In the autocatalysis, hydroxyl radicals possibly transform aromatic moieties to hydroquinone-like moieties, which can reduce iron associated to them. CREDIT: Luca Carena / The University of Torino