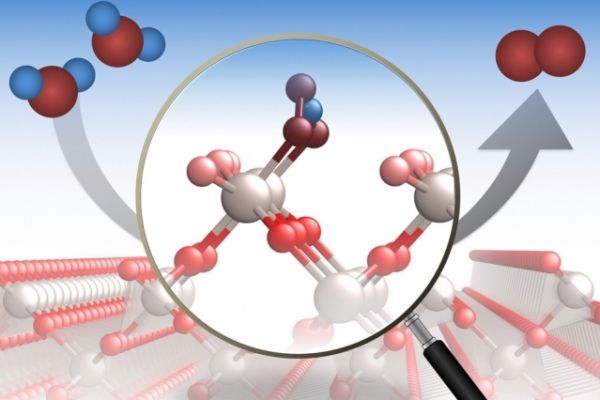
A crystalline compound called ruthenium dioxide is widely used in industrial processes, where it’s particularly important for catalyzing a chemical reaction that splits molecules of water and releases oxygen. But the exact mechanism that takes place on this material’s surface, and how that reaction is affected by the orientation of the crystal surfaces, had never been determined in detail. Now, a team of researchers at MIT and several other institutions has for the first time been able to directly study the process at an atomic level.
The new findings are reported this week in the journal Nature Catalysis, in a paper by MIT Professor Yang Shao-Horn, recent graduate students Reshma Rao, Manuel Kolb, Livia Giordano and Jaclyn Lunger, and 10 others at MIT, Argonne National Laboratory, and other institutions.
The work involved years of collaboration and an iterative process between atom-by-atom computer modeling of the catalytic process, and precision experiments including some using a unique synchrotron X-ray facility at Argonne, which allows atomic-scale probing of the material’s surface.
Read more at Massachusetts Institute of Technology
Image: Oxygen evolution reactions are important in a variety of industrial processes. A new study provides a detailed analysis of the process at a molecular level. As illustrated here, the researchers analyzed how molecules of water (H2O, left) are catalyzed by specific locations on a surface of ruthenium dioxide (center) to form molecules of oxygen (O2, right).
Courtesy of the researchers