A team of researchers at the Department of Energy’s Oak Ridge and Argonne national laboratories has performed the first room-temperature X-ray measurements on the SARS-CoV-2 main protease — the enzyme that enables the virus to reproduce.
The X-ray measurements mark an important first step in the researchers’ ultimate goal of building a comprehensive 3D model of the enzymatic protein. The model will be used to advance supercomputing simulations aimed at finding drug inhibitors to block the virus’s replication mechanism and help end the COVID-19 pandemic. Their research results are publicly available and have been published in the journal Nature Communications.
SARS-CoV-2 is the virus that causes the disease COVID-19. The virus reproduces by expressing long chains of proteins that must be cut into smaller lengths by the protease enzyme.
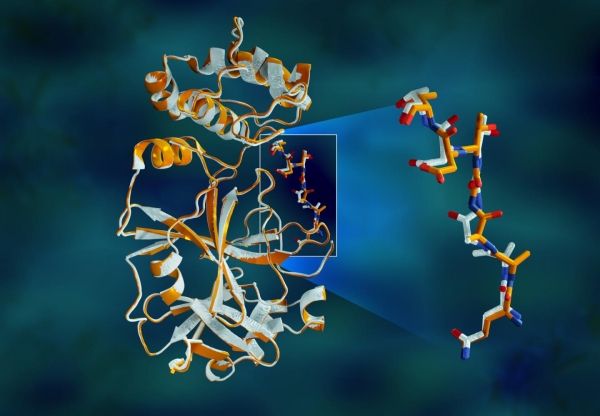
“The protease is indispensable for the virus life-cycle. The protein is shaped like a valentine’s heart, but it really is the heart of the virus that allows it to replicate and spread. If you inhibit the protease and stop the heart, the virus cannot produce the proteins that are essential for its replication. That’s why the protease is considered such an important drug target,” said ORNL’s Andrey Kovalevsky, corresponding author. While the structure is known from cryogenically preserved crystals, “This is the first time the structure of this enzyme has been measured at room temperature, which is significant because it’s near the physiological temperature where the cells operate.”
Read more at DOE/Oak Ridge National Laboratory
Image: Overlapping X-ray data of the SARS-CoV-2 main protease shows structural differences between the protein at room temperature (orange) and the cryogenically frozen structure (white). (Credit: Jill Hemman/ORNL, U.S. Dept. of Energy)