When a bicycle gets wet in the rain, the frame and chain become corroded or rusty which shorten the life of the bike. Oil needs to be regularly applied to prevent this from happening. Battery cells are devices that create electrical energy through moving electrons by triggering oxidation and reduction reactions separately. But they also corrode when exposed to oxygen. Can these cells also be greased to prevent rusting?
A research team led by Professor Yong-Tae Kim and doctoral student Sang Moon Jung of Materials Science and Engineering at POSTECH used a catalyst (Pt/HxWO3) that combines platinum and hydrogen tungsten bronze to solve the corrosion in fuel cells that occur when hydrogen cars are shut down. The catalyst, recently introduced in Nature Catalysis – a sister journal of Nature – has been shown to promote hydrogen oxidation and selectively suppress oxygen reduction reactions (ORR).
As eco-friendly hydrogen cars become more common, the race for research and development for improving fuel cell performance – the heart of hydrogen cars – is getting fierce around the world. The performance of automotive fuel cells are severely low owing to their intermittent shut-downs compared to power-generating fuel cells that do not stop once started. This is because when ignition is turned off, the ORR occurs as air is temporarily introduced into the anode, and corrosion of the cathodic components accelerates as the potential of cathod surges instantaneously.
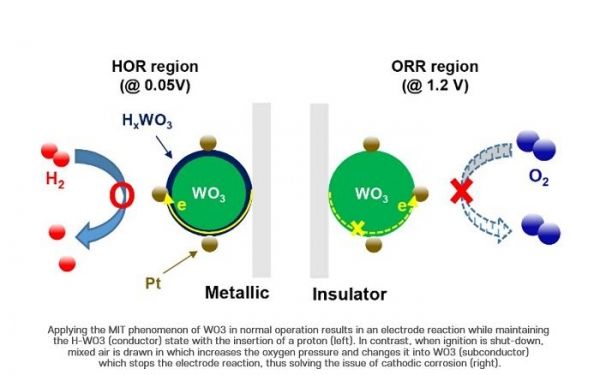
The research team focused on the Metal Insulator Transition (MIT) phenomenon, which can selectively change the conductivity of materials depending on the surrounding environment, to solve the problem of durability degradation in automotive fuel cells.
Read more at Pohang University of Science & Technology (POSTECH)
Image: Applying the MIT phenomenon of W03 in normal operation results in an electrode reaction while maintaing the H-W03 (Conductor) state with the insertion of a proton (left). In contrast, when ignition is shut-down, mixed air is drawn in which increases the oxygen pressure and changes it into W03 (subconductor) which stops the electrode reaction, thus solvoing the issue of cathodic corrosion (right). (Credit: Yong-Tae Kim (POSTECH))