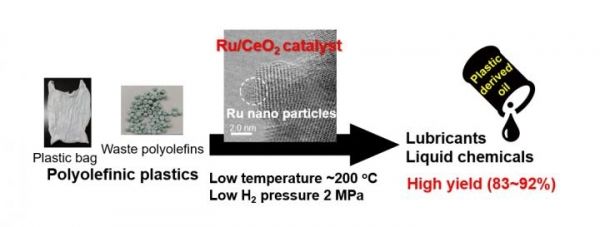
For the first time, researchers have used a novel catalyst process to recycle a type of plastic found in everything from grocery bags and food packaging to toys and electronics into liquid fuels and wax.
The team published their results on Dec. 10, 2020 in Applied Catalysis B: Environmental.
“Plastics are essential materials for our life because they bring safety and hygiene to our society,” said paper co-authors Masazumi Tamura, associate professor in the Research Center for Artificial Photosynthesis in the Advanced Research Institute for Natural Science and Technology in Osaka City University, and Keiichi Tomishige, professor in the Graduate School of Engineering in Tohoku University. “However, the growth of the global plastic production and the rapid penetration of plastics into our society brought mismanagement of waste plastics, causing serious environmental and biological issues such as ocean pollution.”
Polyolefinic plastics — the most common plastic — have physical properties that make it difficult for a catalyst, responsible for inducing chemical transformation, to interact directly with the molecular elements to cause a change. Current recycling efforts require temperatures of at least 573 degrees Kelvin, and up to 1,173 degrees Kelvin. For comparison, water boils at 373.15 degrees Kelvin, and the surface of the Sun is 5,778 degrees Kelvin.
Read more at: Osaka City University
Graphical abstract (Photo Credit: Tamura/Osaka City University)