Researchers in the Oregon State University College of Engineering have developed a battery anode based on a new nanostructured alloy that could revolutionize the way energy storage devices are designed and manufactured.
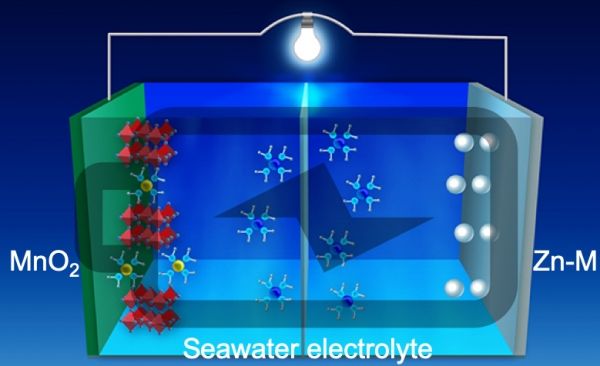
The zinc- and manganese-based alloy further opens the door to replacing solvents commonly used in battery electrolytes with something much safer and inexpensive, as well as abundant: seawater.
Findings were published today in Nature Communications.
“The world’s energy needs are increasing, but the development of next-generation electrochemical energy storage systems with high energy density and long cycling life remains technically challenging,” said Zhenxing Feng, a chemical engineering researcher at OSU. “Aqueous batteries, which use water-based conducting solutions as the electrolytes, are an emerging and much safer alternative to lithium-ion batteries. But the energy density of aqueous systems has been comparatively low, and also the water will react with the lithium, which has further hindered aqueous batteries’ widespread use.”
Read more at Oregon State University
Image: Seawater battery. CREDIT: Oregon State University