Scientists at Tokyo Institute of Technology (Tokyo Tech), Tohoku University, National Institute of Advanced Industrial Science and Technology, and Nippon Institute of Technology, demonstrated by experiment that a clean electrolyte/electrode interface is key to realizing high-capacity solid-state lithium batteries. Their findings could pave the way for improved battery designs with increased capacity, stability, and safety for both mobile devices and electric vehicles.
Liquid lithium-ion batteries are everywhere, being found in the majority of everyday mobile devices. While they possess a fair share of advantages, liquid-based batteries carry notable risks as well. This has become clear to the public in recent years after reports of smartphones bursting into flames due to design errors that caused the battery's liquid electrolyte to leak and catch fire.
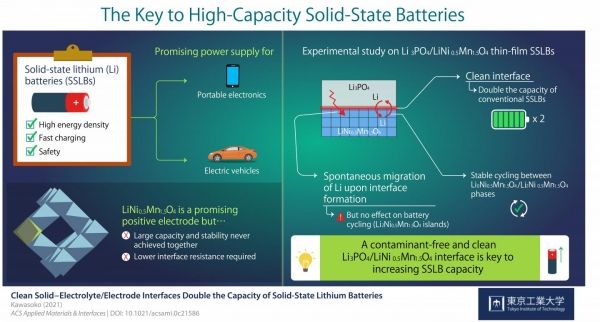
Other disadvantages such as fabrication cost, durability, and capacity, led scientists to look into a different technology: solid-state lithium batteries (SSLBs). SSLBs comprise solid electrodes and a solid electrolyte that exchange lithium (Li) ions during charging and discharging. Their higher energy density and safety make SSLBs very powerful sources.
However, there are still many technical challenges preventing SSLBs' commercialization. For the current study, researchers conducted a series of experiments and gained insight that could take SSLBs' performance to the next level. Professor Taro Hitosugi from Tokyo Tech, who led the study, explains their motivation: "LiNi0.5Mn1.5O4 (LNMO) is a promising material for the positive electrode of SSLBs because it can generate comparatively higher voltages. In this study, we showed battery operations at 2.9 and 4.7 V, and simultaneously achieved large capacity, stable cycling, and low resistance at the electrolyte/electrode interface."
Read more at Tokyo Institute of Technology
Image: The key to High-Capacity Solid-State Batteries (Credit: Taro Hitosugi)