Hydrogen will be needed in large quantities as an energy carrier and raw material in the energy system of the future. To achieve this, however, hydrogen must be produced in a climate-neutral way, for example through so-called photoelectrolysis, by using sunlight to split water into hydrogen and oxygen. As photoelectrodes, semiconducting materials are needed that convert sunlight into electricity and remain stable in water. Metal oxides are among the best candidates for stable and inexpensive photoelectrodes. Some of these metal oxides also have catalytically active surfaces that accelerate the formation of hydrogen at the cathode or oxygen at the anode.
Why is rust not much better?
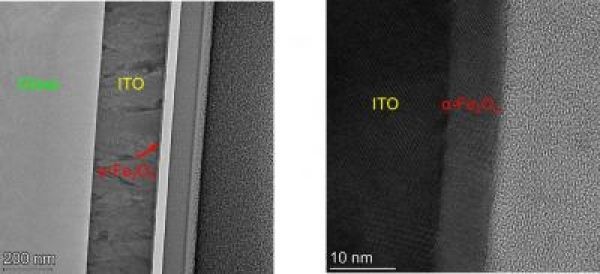
Research has long focused on haematite (α-Fe2O3), which is widely known as rust. Haematite is stable in water, extremely inexpensive and well suited as a photoanode with a demonstrated catalytic activity for oxygen evolution. Although research on haematite photoanodes has been going on for about 50 years, the photocurrent conversion efficiency is less than 50% of the theoretical maximum value. By comparison, the photocurrent efficiency of the semiconductor material silicon, which now dominates almost 90% of the photovoltaic market, is about 90% of the theoretical maximum value.
Scientists have puzzled over this for a long time. What exactly has been overlooked? What is the reason that only modest increases in efficiency have been achieved?
Read more at Helmholtz-Zentrum Berlin Für Materialien und Energie
Image: Rust would be an extremely cheap and stable photoelectrode material to produce green hydrogen with light. But the efficiency is limited. The TEM image shows a photoanode containing a thin photoactive layer of rust. (Credit: Technion)