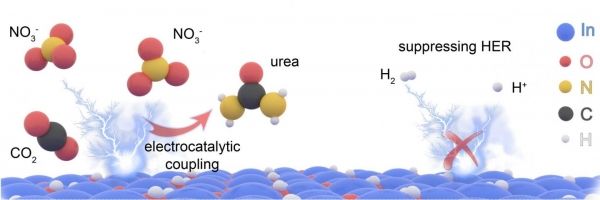
Urea is a critical element found in everything from fertilizers to skin care products. Large-scale production of urea, which is naturally a product of human urine, is a massive undertaking, making up about 2% of global energy use and emissions today.
For decades, scientists and engineers have sought to make this process more energy efficient as demand for fertilizer grows with increased population. An international research team that includes scientists and engineers from The University of Texas at Austin has devised a new method for making urea that is more environmentally friendly than today’s process and produces enough to be competitive with energy-intensive industrial methods.
Making urea today involves a two-step thermal process that requires high levels of heat and pressure under controlled harsh environments. But this new process requires just one step and relies on a concept called electrocatalysis that uses electricity — and potentially sunlight — to trigger chemical reactions in a solution at room temperature in ambient conditions.
Read more at: University of Texas at Austin
The electrocatalytic reaction between these building blocks could make urea production much more energy efficient. (Photo Credit: The University of Texas at Austin)