Since the early days of the COVID pandemic, scientists have aggressively pursued the secrets of the mechanisms that allow severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) to enter and infect healthy human cells.
Early in the pandemic, University of California San Diego’s Rommie Amaro, a computational biophysical chemist, helped develop a detailed visualization of the SARS-CoV-2 spike protein that efficiently latches onto our cell receptors.
Now, Amaro and her research colleagues from UC San Diego, University of Pittsburgh, University of Texas at Austin, Columbia University and University of Wisconsin-Milwaukee have discovered how glycans—molecules that make up a sugary residue around the edges of the spike protein—act as infection gateways.
Published August 19 in the journal Nature Chemistry, a research study led by Amaro, co-senior author Lillian Chong at the University of Pittsburgh, first author and UC San Diego graduate student Terra Sztain and co-first author and UC San Diego postdoctoral scholar Surl-Hee Ahn, describes the discovery of glycan “gates” that open to allow SARS-CoV-2 entry.
Read more at University of California - San Diego
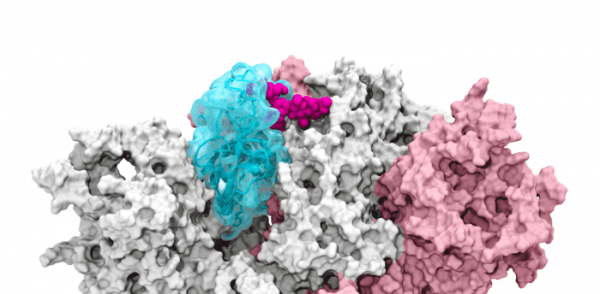
Image: The glycan gate opens: Supercomputing-driven simulations depict the glycan N343 (magenta) acting as a molecular crowbar to pry open the SARS-CoV-2 spike’s receptor binding domain, or RBD (cyan), from a “down” to an "up" position. (Credit: Terra Sztain, Surl-Hee Ahn, Lorenzo Casalino (Amaro Lab, UC San Diego))