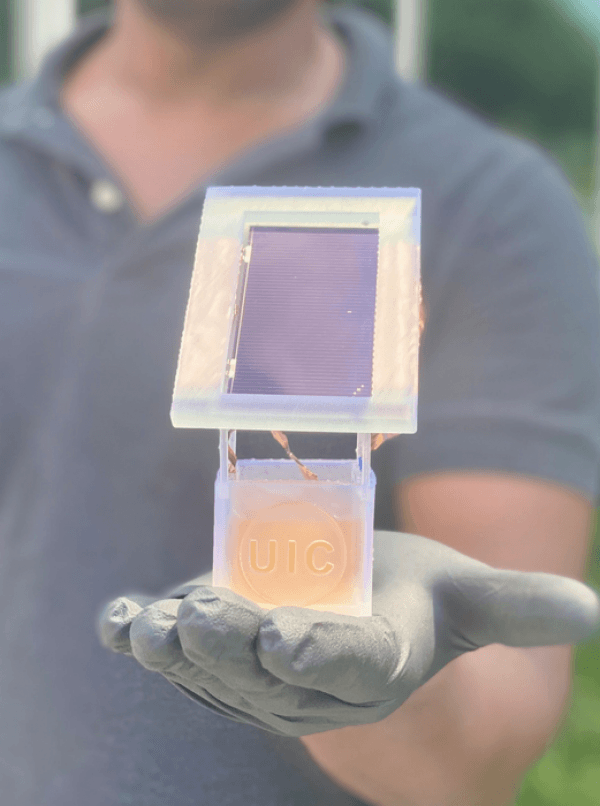
Engineers at the University of Illinois Chicago have created a solar-powered electrochemical reaction that not only uses wastewater to make ammonia — the second most-produced chemical in the world — but also achieves a solar-to-fuel efficiency that is 10 times better than any other comparable technology.
Their findings are published in Energy & Environmental Science, a top journal for research at the intersection of energy delivery and environmental protections.
“This technology and our method have great potential for allowing on-demand synthesis of fertilizers and could have an immense impact on the agricultural and energy sectors in developed and developing countries, and on efforts to reduce greenhouse gases from fossil fuels,” said lead researcher Meenesh Singh, assistant professor of chemical engineering at the UIC College of Engineering.
Ammonia, a combination of one nitrogen atom and three hydrogen atoms, is a key compound of fertilizers and many manufactured products, like plastics and pharmaceuticals. Current methods to make ammonia from nitrogen require enormous amounts of heat, generated by burning fossil fuels, to break the strong bonds between nitrogen atoms so they can bind to hydrogen. This century-old process produces a substantial fraction of global greenhouse gas emissions, which are a driving force of climate change.
Read more at University of Illinois at Chicago
Image: UIC researchers create a sustainable electrochemical system in which a solar cell is attached to a well holding a liquid solution. When charged, nitrates from wastewater in the liquid solution are converted to ammonia. (Photo Credit: Meenesh Singh/UIC)