Scientists at Lawrence Berkeley National Laboratory (Berkeley Lab) have demonstrated a new technique, modeled after a metabolic process found in some bacteria, for converting carbon dioxide (CO2) into liquid acetate, a key ingredient in “liquid sunlight” or solar fuels produced through artificial photosynthesis.
The new approach, reported in Nature Catalysis, could help advance carbon-free alternatives to fossil fuels linked to global warming and climate change.
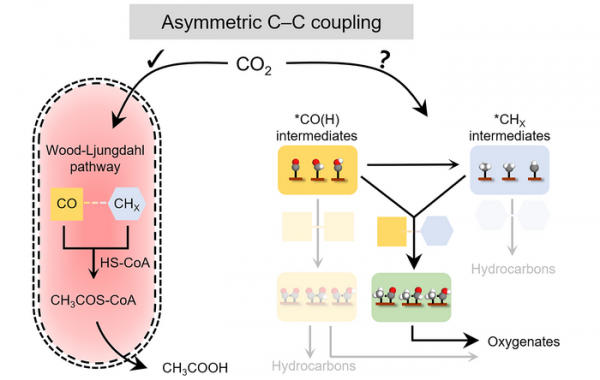
The work is also the first demonstration of a device that mimics how these bacteria naturally synthesize acetate from electrons and CO2.
“What’s amazing is that we learned how to selectively convert carbon dioxide into acetate by mimicking how these little microorganisms do it naturally,” said senior author Peidong Yang, who holds titles of senior faculty scientist in Berkeley Lab’s Materials Sciences Division and professor of chemistry and materials science and engineering at UC Berkeley.
Read more at: Lawrence Berkeley National Laboratory
For decades, researchers have known that a metabolic pathway in some bacteria allows them to digest electrons and CO2 to produce acetate, a reaction driven by the electrons. The pathway breaks CO2 molecules down into two different or “asymmetric” chemical groups: a carbonyl group (CO) or a methyl group (CH3). Enzymes in this reaction pathway enable the carbons in CO and CH3 to bond or “couple,” which then triggers another catalytic reaction that produces acetate as the final product. Now, Berkeley Lab scientists have demonstrated a new technique, modeled after this bacterial metabolic pathway, to convert carbon dioxide into solar fuels through artificial photosynthesis. (Photo Credit: Chubai Chen/Berkeley Lab; courtesy of Nature Catalysis)