In recent years, environmental problems caused by global warming have become more apparent due to greenhouse gases such as CO2. In natural photosynthesis, CO2 is not reduced directly, but is bound to organic compounds which are converted to glucose or starch. Mimicking this, artificial photosynthesis could reduce CO2 by combining it into organic compounds to be used as raw materials, which can be converted into durable forms such as plastic.
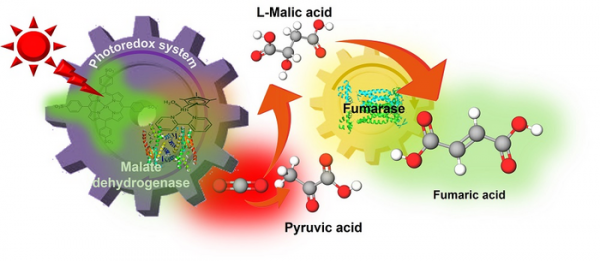
A research team led by Professor Yutaka Amao from the Research Center for Artificial Photosynthesis and graduate student Mika Takeuchi, from the Osaka Metropolitan University Graduate School of Science, have succeeded in synthesizing fumaric acid from CO2, a raw material for plastics, powered—for the first time—by sunlight. Their findings were published in Sustainable Energy & Fuels.
Fumaric acid is typically synthesized from petroleum, to be used as a raw material for making biodegradable plastics such as polybutylene succinate, but this discovery shows that fumaric acid can be synthesized from CO2 and biomass-derived compounds using renewable solar energy.
Read more at: Osaka Metropolitan University
Using sunlight to power the photoredox system pyruvic acid and CO¬2 are converted into fumaric acid, by malate dehydrogenase and fumarase. (Photo Credit: Yutaka Amao, Osaka Metropolitan University)