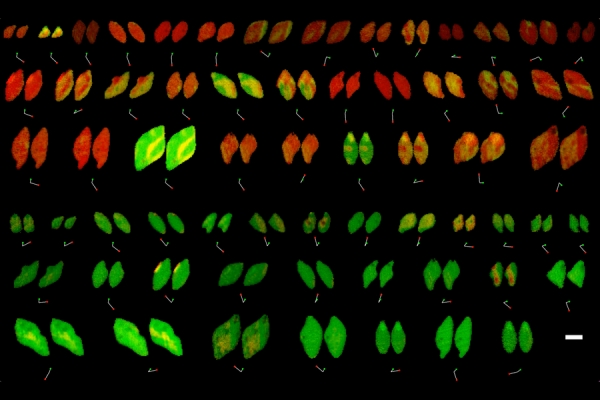
By mining data from X-ray images, researchers at MIT, Stanford University, SLAC National Accelerator, and the Toyota Research Institute have made significant new discoveries about the reactivity of lithium iron phosphate, a material used in batteries for electric cars and in other rechargeable batteries.
The new technique has revealed several phenomena that were previously impossible to see, including variations in the rate of lithium intercalation reactions in different regions of a lithium iron phosphate nanoparticle.
The paper’s most significant practical finding — that these variations in reaction rate are correlated with differences in the thickness of the carbon coating on the surface of the particles — could lead to improvements in the efficiency of charging and discharging such batteries.
“What we learned from this study is that it’s the interfaces that really control the dynamics of the battery, especially in today’s modern batteries made from nanoparticles of the active material. That means that our focus should really be on engineering that interface,” says Martin Bazant, the E.G. Roos Professor of Chemical Engineering and a professor of mathematics at MIT, who is the senior author of the study.
Read more at Massachusetts Institute of Technology
Image: By mining X-ray images, MIT researchers have made significant new discoveries about the reactivity of lithium iron phosphate, a material used in batteries for electric cars and in other rechargeable batteries. In each pair pictured, actual particles are on the left and the researchers’ simulations are on the right. Credits: Courtesy of the researchers