Of the four states of matter, gases are the hardest to pin down. Gas molecules move quickly and wildly and don’t like to be confined. When confined, heat and pressure build in the container, and it doesn’t take long before the gas blows the lid off the place, literally. Luckily, gases are superficial. Provide them with an attractive internal surface area, and they’ll pin themselves down in no time. No, it’s not love at first sight, it’s adsorption.
“Adsorption is the processes of gas pinning to the surface of another material—the inside walls of a container, for example,” says Chris Wilmer, assistant professor in Pitt’s Department of Chemical and Petroleum Engineering. “When adsorption occurs, the gas molecules stop bumping into each other, reducing pressure. So, by increasing a container’s internal surface area, we can store more gas in less space.”
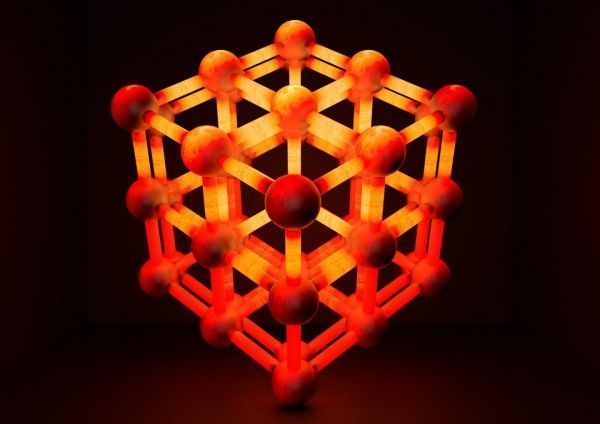
Dr. Wilmer directs the Hypothetical Materials Lab, where he and his research group develop new ways to store, separate, and transport gases. They recently published their study “Thermal Transport in Interpenetrated Metal-Organic Frameworks” (DOI: 10.1021/acs.chemmater.7b05015) in the American Chemistry Society Journal Chemistry of Materials. The issue’s cover also featured an image designed by Kutay Sezginel, a chemical engineering graduate student in Dr. Wilmer’s Lab. It depicted interpenetrated metal organic frameworks or MOFs.
MOFs are a promising class of porous materials, made of metal clusters bound to organic molecules. Discovered fewer than two decades ago, MOFs help rein in gases because their porous nanostructure has an extremely high surface area and can be custom engineered to be particularly sticky to certain gas molecules. MOFs are used for a variety of functions including gas storage, gas separation, sensing, and catalysis.
Read more at University of Pittsburgh
Image: This is an idealized interpenetrated MOF structure. The entangled MOF can dissipate heat roughly two times faster than the constituent MOFs could separately, potentially enabling more efficient gas storage. (Credit: Swanson School of Engineering)